
Condition Monitoring Solutions
Smart, Connected Cryogenic Storage
Condition monitoring provides continuous visibility of critical cryogenic storage conditions, helping laboratories protect valuable biological samples, detect issues early, and maintain confidence in sample integrity and compliance.
Confidence Without Compromise
Condition monitoring enables continuous visibility of critical cryogenic storage conditions, helping protect valuable biological materials through near real‑time insights, automated alerts, and a secure digital record that supports compliance. Integrated directly into MVE dewars, it transforms storage vessels into intelligent, connected assets—giving users greater confidence, control, and operational efficiency.

Seamless Integration
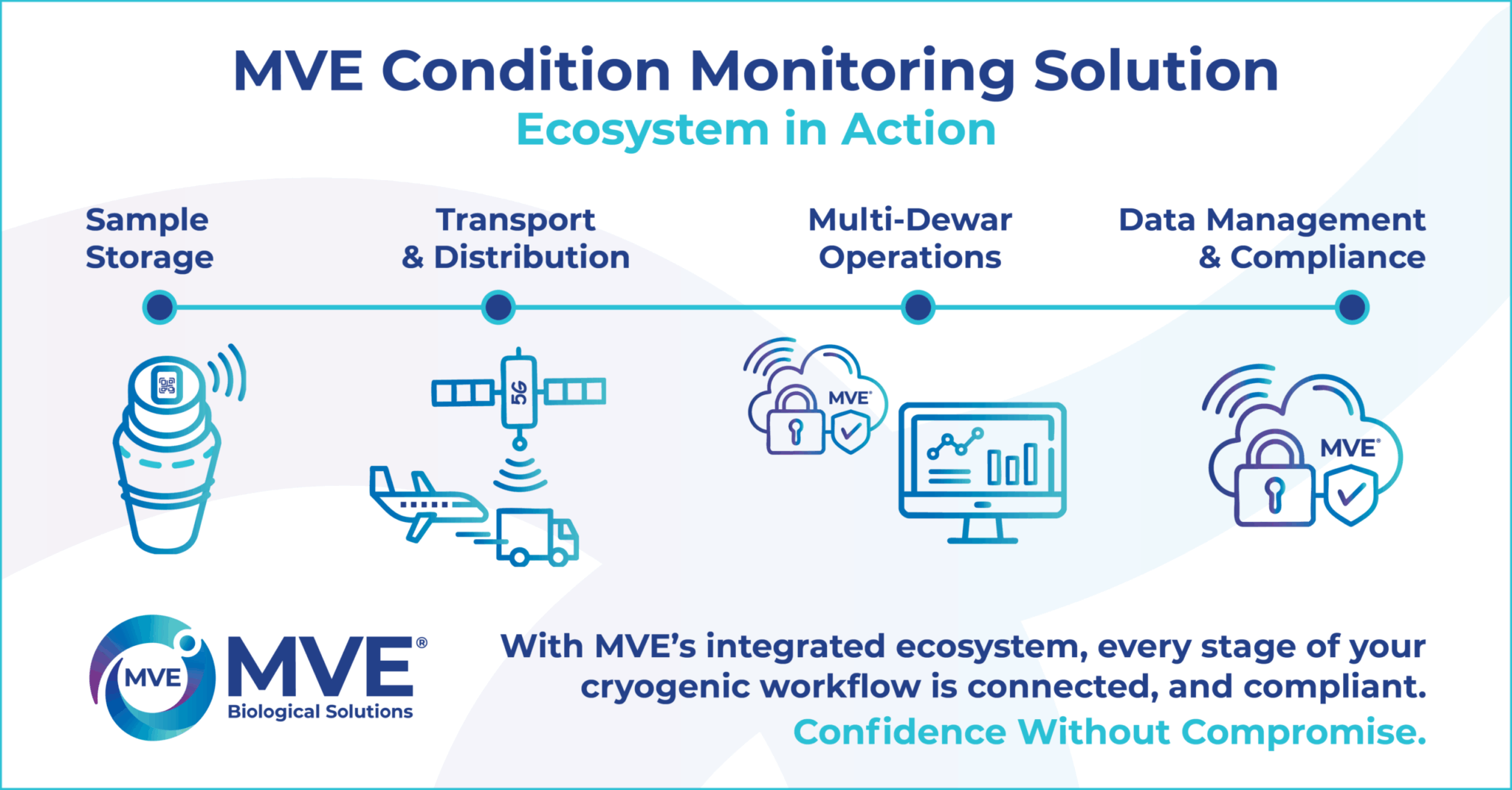
MVE’s Condition Monitoring is designed as a fully integrated ecosystem, combining sensors, connectivity, and secure cloud access directly within the dewar.
Unlike third party devices that require external attachments or manual setup, MVE’s solution is engineered to work in harmony with the dewar itself. This built in integration delivers more reliable data, reduces the risk of lost or mismatched records, and minimises staff workload by capturing and displaying information at the source. The result is a single, trusted system that safeguards sample integrity while streamlining operations, compliance, and logistics—ensuring every data point is securely captured without gaps.
Built in monitoring is available on MVE SC 4/2 V and SC 4/3 V dewars through:
- CryoBeacon (On Demand Monitoring): Bluetooth enabled monitoring with QR code access to historical data
- SmartTag (Near Real Time Monitoring): 5G LTE connectivity for continuous data transmission during storage or transport
- MVECloud & App: A secure, FDA 21 CFR Part 11 compliant platform providing dashboards, alerts, and reporting

Introducing the MVECloud
MVECloud is a secure, centralised platform that gives users anytime access to condition monitoring data for samples in storage or in transit. Through a web portal or mobile app, users can view near real‑time status, historical trends, and complete digital records, with multi‑channel alerts ensuring critical updates are never missed. Fully FDA 21 CFR Part 11 compliant, MVECloud supports data integrity and simplifies regulatory compliance with audit‑ready reporting—helping teams maintain full confidence in the chain of custody across global operations.
Specifications
| MVE CryoBeacon | MVE SmartTag | |
|---|---|---|
| Monitoring Type | On-Demand | Near Real-Time |
| Connectivity | BLE / QR Scan | 5G LTE CAT M1 |
| Sensors | Temp, humidity | Temp, orientation, shock, light, acceleration, GPS |
| Battery Life | 1 year | Up to 120 days; Rechargeable with Micro-USB |
| Ideal Use Case | Long-term storage, global shipments, periodic checks | Global shipments, near real-time oversight |
Arrange to speak to
an expert
Complete the form below and one of our experts will be in touch.
Download our
brochure



